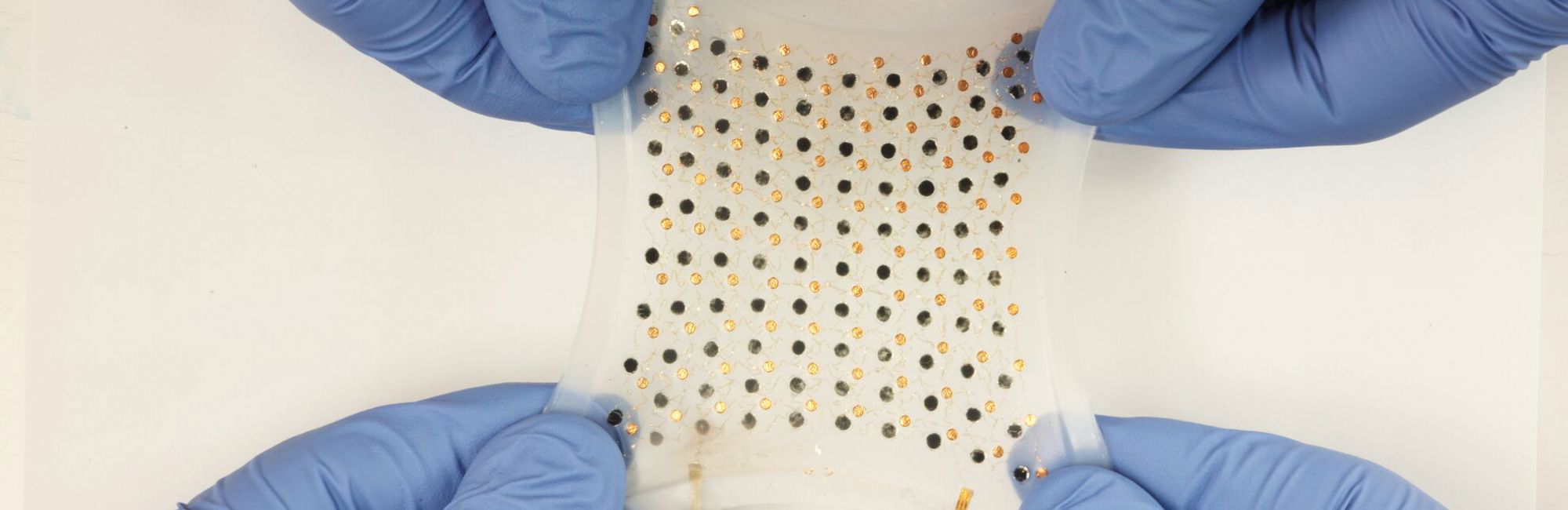
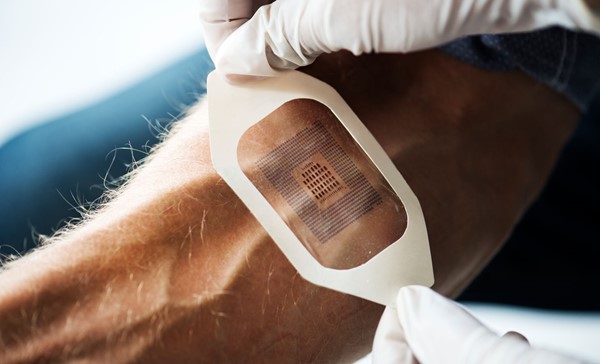
Medical technology is a broad field, encompassing many types of instruments, apparatus, implants, software, and other articles. The World Health Organization estimates there to be two million different kinds of medical devices on the market, categorised into more than 7,000 generic groups.
Many different materials are used to manufacture this array of devices – from metals to thermoplastics, ceramics, tissue-engineered medical products, and biologic materials. Each material has its individual advantages for specific applications, but one material stands out for another reason: versatility.
Medical-grade silicone
Silicone finds applications across most fields of medical technology since it offers excellent physical properties and resistance to chemical and thermal degradation. Perhaps most importantly, silicone is chemically inert, which is what guarantees its biocompatibility.
While the medical sector’s earliest use of the synthetic polymer was to coat glassware and needles, over the past 60 years silicone companies have introduced a wide range of medical-grade materials to the market, with varying properties and levels of hardness. Liquid silicone rubber is one of the more modern concepts. When introduced, it transformed production possibilities by enabling a more cost-effective method of processing called liquid injection moulding.
According to Michael Goglia, Healthcare Market Manager for Americas at Elkem Silicones, it is a combination of silicone’s processability and outstanding biocompatibility which have made it such a popular material in the field.
"Anytime a material is implanted into the human body it can sometimes illicit a “Foreign Body Reaction” or “FBR” silicone which produces inflammation around the implanted material, Silicones have shown over the course of historical studies to be excellent at deterring this response.,” Goglia explains. “It also has the ability to bond to other substrates, such as thermoplastics, so it can be used in combination with other materials to provide both a soft and rigid component to the device."
Another factor in silicone’s versatility is the range of cure mechanisms available to manufacturers, he adds, listing examples such as moisture cure at ambient temperature, thermal cure which accelerates the process through elevated temperatures, and even UVC systems that use light.
“In addition to that, silicones can be 3D printed today,” notes Goglia. “You can prototype with 3D printing and then convert that to actual production using traditional moulding methods.”
All of this has opened up a world of possibility for medical OEMs, enabling them to take advantage of silicone’s numerous benefits and broad processing parameter ranges. Its biocompatibility has even made it safe to use in long-term implanted devices such as stents, pacemakers, and implantable drug delivery devices.
Being soft to the touch and conformable, there are also silicones used to enhance patient comfort in pressure beds (prosthetics and orthotics), ventilation masks, and atraumatic adhesives. The material’s hydrophobicity makes it an effective lubricious coating for surgical tools and syringes, meanwhile this water repellence helps ensure bacteria resistance too – a huge benefit for the medical industry.
Silicone also has an important application in diagnostic devices that require a battery and internet connection to continuously monitor aspects of patient health in real-time. Silicone is a key material to make this combination work due to its ability to be moulded at lower temperatures than traditional plastics and adhere to multiple substrates either mechanically or through chemical bonds.
It’s easy to see why silicone, in all its various forms, has become a go-to for medical devices. To explore further advantages, let’s take a more detailed look at the different types of silicone used by the industry.
Innovative medical device trends enabled by silicone
Unleashing Medtech innovation, getting through regulatory & supply hurdles
Types of medical-grade silicone
Liquid silicone rubber (LSR) is a popular choice. It is typically used within injection moulding machines, where it cures completely and quickly to create complex parts with fine detail, tight tolerances and low shrink rates.
“This has become the material of choice for medical engineers as it is scalable, cost effective, and widely recognised for its excellent biocompatible properties,” says Goglia. “Small-scale design can be done in a benchtop environment on a 3D-printed mould and scale all the way up to a fully automated multi-cavity injection environment running 24/7.”
High consistency rubber (HCR) has a higher viscosity and is better suited to high-strength moulding and extrusion processes. Typical applications include pharmaceutical transfer hose and gasketing, fluid handling and feeding tubes, as well as high-strength balloons that can be used in catheters. Overall, the performance characteristics of LSR and HCR can be similar and each material boasts a huge variety of medical applications. The choice between them usually comes down to required physical characteristics and manufacturing method. Elkem Silicones produces HCR-grade materials globally in the US, Europe and Asia, with various grades meeting differentiating performance criteria.
Other forms of silicone have adhesive properties, making them a great sealing solution for applications where high-strength elastic bonds between substrates are needed. These materials come in both moisture cure one-part systems and two-part addition cure systems. They are excellent at adhering silicone to silicone as well as adhering silicone to various other widely used medical substrates safely and effectively.
These same properties add another medical application to silicone’s long list: skin adhesion. Used across wound care, medical tape, and transdermal drug delivery patch products, silicone adhesives such as Silbione™ Silicone Skin Adhesives provide reliable yet non-traumatic and hypoallergenic adhesion to skin. They also allow media to be removed and replaced over a period of time allowing for greater patient flexibility.
Medical-grade room temperature vulcanising (RTV) silicones comprise a two-part system that can be easily mixed and cured into soft yet durable elastomers. Ideal for prosthetic liners, orthotic inserts, bandages, and shoe insoles, RTVs provide comfort and biocompatibility in the manufacturing of soft parts in contact with a patient’s skin.
Silicones are also available in gels and fluids. Gels are very soft elastomers used in devices like cushions and breast forms. Silicone fluids are made from polydimethylsiloxanes fluids, emulsions and RTV lubricants, and are used to coat and lubricate parenteral packaging components such as vials and syringes, as well as surgical tools.
Since silicone comes in so many different forms and has a list of advantages to offer, it is common for medical device designers to make use of various types of silicone within a single device. Taking a wearable device as an example, Philipp Schattling, Elkem’s project leader for diagnostics and long-term medical implants, explains: “Wearable devices consist of many different components and many different building blocks, and every building block may require different properties and performance characteristics.
“This can be a challenge because usually with just one technology you cannot address each and every requirement. The benefit of silicones is that they cover a broad range of different technologies. We have the glue, the sealant, the adhesive, the LSR for the housing, and the HCR for the tubing.”
For a full selection of medical-grade silicone technologies, medical device OEMs can explore Elkem Silicones’ Silbione™ range of LSR, HCR, silicone adhesive, silicone skin adhesive, RTV, silicone gel, and silicone fluid products. The materials offer exceptional physical properties to help customers differentiate their products and ensure precise final performance. They are also designed with ease of processing in mind, ensuring improved productivity and enhanced competitiveness for customers.
Innovative medical device trends enabled by silicone
Silicone materials has been taken medical devices design to another level and is set to accelerate innovations in this area.