What is silicone?
Silicone is an inert synthetic compound that come in a variety of forms (oil, rubber, resin). Typically, heat-resistant and rubber-like, they are present in sealants, adhesives, lubricants, medical applications, cookware and insulation. Silicone is a polymer that contains silicon, combined with carbon, hydrogen and oxygen and, in some cases, other elements.
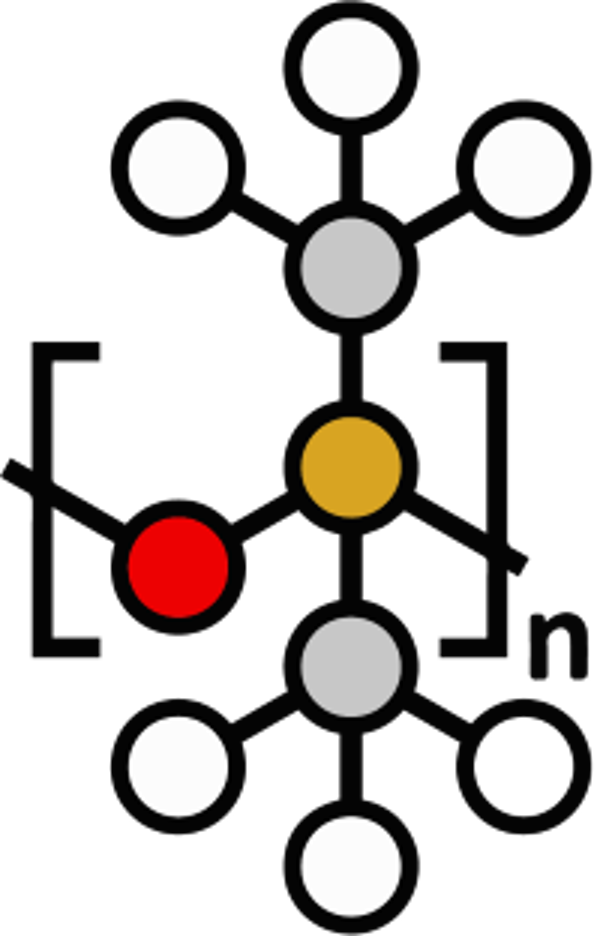
Chemical Structure of silicone
The basic structure of silicone is made up of polyorganosiloxanes, where silicon atoms are linked to oxygen to create the «siloxane» bond. The remaining valences of silicon are linked to organic groups, mainly methyl groups (CH3): Phenyl, vinyl or hydrogen.
Discover our latest video "What are Silicones?":
Silicone Properties
Silicone, due to its polysiloxane, provide several advantages:
- Thermal stability (from -80 °C to 250 °C)
- Resistance to natural ageing (oxidation, UV)
- Resistance to fire, low emission of smoke and toxic fumes, self-protection, ceramization of ashes
- Low surface energy
- Good wetting on many substrates
- Hydrophobia (beading effect)
- Release or adhesion properties, according to need
- Exceptional harmlessness for a wide range of applications
- Biocompatibility, well-suited for food contact and medical applications
- Safe and comfortable skin contact
- Flexible chain of up to -100 °C for enhanced lubrication and gas permeability performance
- Easy processing, excellent spread and coating capabilities
- Available in a variety of forms – fluids, liquid silicone elastomers (LSR) and high-consistency rubbers (HCR)
![]()
Discover the different silicone technologies
Contact us
Take your business to the next level by partnering-up with a global leading material manufacturer.